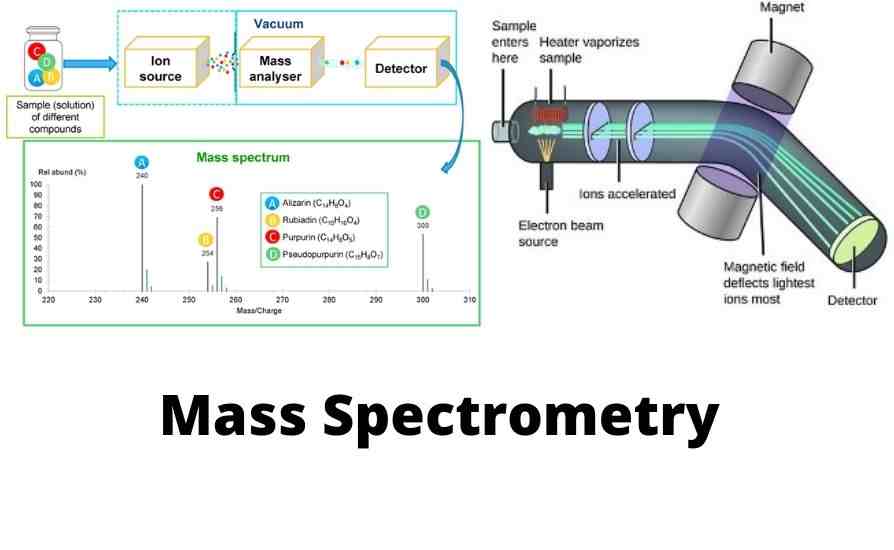
What Is Mass Spectrometry or Mass Spectroscopy?
Mass spectroscopy is an analytical technique that produces mass spectrums. It identifies chemical materials by sorting their gaseous ions or charged particles in a unified electric and magnetic field according to the mass-to-charge (m/z) ratios of ions. The instruments that employ this technique are called mass spectrometers or mass spectrographs. Both operate based on the principle that moving ions get deflected while traveling through a mixed electric and magnetic field. In mass spectrometer charged particles or ions are detected electrically, while in mass spectrograph they are detected by nonelectrical means such as photographic. The term mass spectroscope covers both kinds of instruments. At present-day electrical detectors are most commonly used in mass spectroscopes. Hence the branch is usually referred to as mass spectrometry.
History of Mass Spectrometer
German physicist Wilhelm Wien laid the groundwork of mass spectroscopy in 1898 when he observed that a magnetic field could deflect beams of charged particles. British physicist J.J. Thomson from 1907 to 1913 undertook more elaborated experiments on the discovery of Wilhelm Wien and successfully passed a beam of positively charged ions through a combination of an electrostatic and magnetic field. The origin of the first mass spectrometer can be related to this period. In 1912 J.J. Thomson built the first mass spectrometer. At that time, J.J. Thomson was already famous for his discovery of the electron in 1897. Initially, a mass spectrometer was called a parabola spectrograph. Back then using mass spectrometer J.J. Thomson was able to unearth the very first proof of the existence of nonradioactive isotopes. Since its inception, mass spectrometry has in effect developed into a universal research apparatus delivering plenty of scientific accomplishments including isotope discovery, atomic weight determination, characterization of elements, molecular structure characterization. For much of the 20th-century, mass spectrometer undoubtedly emerged as the most valuable and complex type of instrument for many areas of science and industry. Though the educated public class somewhat remained unfamiliar with mass spectrometer technology.
Basic Constituents of Mass Spectroscopes
There are five basic components of mass spectroscopes, namely:
Each component of a mass spectroscope can be briefly explained as following.
Mass spectrometer works by producing ions from atoms/single molecules then accelerating them through a composite of an electric and magnetic field to the detector. An inert environment is needed to allow ions to fly freely through a noiseless environment. Very low pressure or high vacuum creates an air-free environment reducing noises from volatile compounds in the air. If there are many other atoms/molecules in the flight path, they would collide with ions causing the ions to react, fragment, neutralize, or scatter. In this case, the presence of a high vacuum minimizes the possibility of collision with ions. Hence mass spectrometer will have higher sensitivity.
A sample introduction system is needed to inject the samples to be investigated (the analyte) into the ion source in a high vacuum environment.
The ion source is the part that produces charged particles or gaseous ions from the material under analysis by bombarding it with electrons, for example. Due to electron bombardment, some molecules may even break into charged fragments. Then the produced ions are guided to the analyzer through a combination of electric and magnetic fields.
There are several ionization methods available for mass spectrometry, each one suitable for different types of samples. They can be divided into three umbrella groups gas-phase ionization, desorption ionization, and spray ionization. Each could be further divided as follows.
Gas-Phase Ionization Techniques-
Electron Impact ionization (EI)
Chemical Ionization (CI)
Direct analysis in real time (DART)
Inductively coupled plasma (ICP)
Desorption Ionization Techniques-
Matrix Assisted Laser Desorption Ionization (MALDI)
Fast Atom Bombardment (FAB)
Thermal Ionization
Plasma Ionization
Liquid metal ion sources (LMIS)
Spray Ionization Techniques-
Thermospary
Electrospray ionization (ESI)
Desorption electrospray ionization (DESI)
Atmospheric Pressure Chemical Ionization (APCI)
Atmospheric Pressure Photoionization (APPI)
A mass analyzer is also called a mass filter. It is at the heart of any mass spectrometer. Mass analyzer separates gas-phase molecular ions and their fragments according to ion velocity, mass, and/or mass-to-charge (m/z) ratios by accelerating them and subjecting them through a combined electric and magnetic field. Separated ions are then driven towards the detector for detection and subsequently converted into a digital signal for processing in a data system.
Then the ions and fragments that outlive the journey through the analyzer are focused onto a detector for detection. There are several types of detectors that mass spectrometers use, such as Photoplate detector, Faraday detector, Electron multiplier detector (EM). Among the early detectors are the Daly detector, Cyrodetector, Focal-plane array detector. However, electron multiplier (EM) detectors are the most commonly used ion detectors in modern mass spectrometers because they can produce exceptionally high gain with minimum noise.
Electron Multiplier Detector (EM Detector)- Electron multiplier (EM) detectors work by a mechanism called secondary electron emission (SEM). It has an electrode in a vacuum called a “dynode”. When hit by an ion or electron having adequate kinetic energy, the dynode emits electrons. This emission of electrons from “dynodes” is called “secondary emission”. In an electron multiplier detector, a series of dynodes are threaded in an array so that secondary emissions can occur repeatedly. One electron strikes the first dynode generating a few secondary electrons. These secondary electrons then hit the next dynode. At each step, the number of electrons amplified exponentially. The total number of dynodes determines the expected gain of an electron multiplier detector.
Additionally, mass spectrometer control, operation, and data management are performed by a data system comprising of a computer, mass spectrometer software, and a printer/recorder. The data system performs the tasks of controlling the mass spectrometer, monitoring various parameters, collecting or acquiring data, processing and analyzing the data, generating customized reports, and finally printing/recording reports.
There Are Different Types of Mass Analyzers Used In Mass Spectrometers
At the heart of any mass spectrometer is a mass analyzer. So, the name of mass spectrometers usually comes according to the type of mass analyzer they have. According to the technology of constructions, there are several types of mass analyzers currently available. Some of the fundamental types of mass analyzers that are commonly available are as follows.
Here we put forth our humble effort to briefly discuss the working principle for each type of mass analyzer.
Quadrupole mass analyzer or Quadrupole mass spectrometer (MS) was first introduced in 1953 by two West German physicists Wolfgang Paul and Helmut Steinwedel. It is regarded as the simplest type of mass spectrometer (MS). The name quadrupole MS comes from the fact that it consists of four parallel rods having circular or hyperbolic cross-sections. These rods are situated parallel to the flight paths of ions which a mass spectrometer measures. Each rod is connected to a radio frequency alternating current (RF) source and a fixed direct current source. Quadrupole mass analyzer filters ions according to their mass-to-charge ratio (m/z) as they travel along the axis of charged rods or poles. Because quadrupole mass analyzer filters ion based on their m/z value, they are alternatively referred to as a mass filter. Quadrupole mass analyzers can appear in a variety of forms such as single quadrupole MS (SQMS), triple quadrupole MS (TQMS) consisting of three quadrupole analyzers in tandem, quadrupole time-of-flight MS (QTOFMS), which is essentially a triple quadrupole MS but the third quadrupole analyzer is replaced with a time-of-flight (TOF) mass analyzer.
As the name indicates, a Time-of-flight (TOF) mass analyzer separates ions according to the time it takes for ions to travel down a fixed-length “flight tube” before reaching the detector. According to physics law, lighter ions will travel faster and reach the detector first while heavier ions will travel slower and reach the detector last. Then based on the flight time of ions, Time-of-flight mass spectrometers (TOF-MS) calculate the mass-to-charge ratio (m/z) of ions. At first, Hammer in 1911 capitalized on the idea of calculating the mass of ions based on the time ions take to travel a fixed distance. Later, in 1946 W. E. Stephans solidified the use of Time-of-flight mass spectrometers (TOFMS). Finally, in 1948 A. E. Cameron and D. F. Eggers developed a modern TOFMS with all core components. TOF-MS instruments usually have high ion transmission efficiency, fast data acquisition rate, fast mass scan rate, very low detection limit. Most of the TOFMS instruments employ multichannel plate detectors (MCP), which are highly sensitive having response times in the range of < 1 ns. Time-of-flight mass spectrometers (TOF-MS) can be constructed in a variety of forms such as single-stage TOFMS, multi-stage TOF/TOFMS, hybrid quadrupole-TOFMS (QTOFMS). Both TOF/TOF-MS and QTOFMS variants function as MS/MS.
Also Read: General Technical Overview of Vacuum Ultraviolet Detector for Gas Chromatography – VUV Detector
Ion trap mass analyzer consists of an ion trapping space surrounded by multiple electrodes. Radiofrequency (RF) voltage and DC voltages generate a trapping electric field that traps ions within the trapping space.
Ion trap mass analyzer can manipulate and store ions through multiple processes such as isolation, fragmentation. A trap might have one or more outlets for the ejection of ions. After exiting the trap ions are driven towards the detector according to their mass-to-charge ratio (m/z). Ion trap mass spectrometers can perform multi-staged MS, which is a major advantage. Multistage MS can provide valuable ionic structure information. Ion trap mass analyzers can have various architectures such as linear ion trap (2-D trap), 3-D ion trap, electrostatic trap (Orbitrap), the magnetic field-based trap also referred to as ion cyclotron resonance (ICR). One of the striking features of ion trap mass spectrometers is that they easily integrate with other technologies such as GC and LC.
The efforts of legendary Nobel Prize winner Wolfgang Paul in the early 1950s led to the invention of the basics of a quadrupole ion trap (QIT) mass analyzer. He developed QIT mass analyzer simultaneously with a quadrupole mass analyzer. QIT mass analyzer acts as a 3-D ion storage device that may dynamically store or confine charged particles, or gaseous ions for a long time. It incorporates three hyperbolic electrodes: a ring electrode and two endcap electrodes at the entrance and exit. When appropriate voltages are applied to these three electrodes, they cause the formation of a cavity of trapping potential, which can trap ions. Radiofrequency (RF) voltages are to the ring electrodes producing a 3D quadrupole electric field within the trapping space. The trapping potential switches at radio frequency (RF) due to that the trap is also referred to as "radio frequency" traps. The quadrupolar field constantly oscillates the trapped ions. The motion of the oscillating ions depends on the voltages applied and their respective mass-to-charge (m/z) ratios. When ion-trapping voltages are modified, ions are ejected mass-selectively in increasing order of their m/z from the cavity of trapping potential through the endcap. The flood of ions is then directed to strike a detector which generates a mass spectrum. The principle of trapping and ejection makes quadrupole ion trap (QIT) mass analyzers fitting to perform multi-stage mass spectrometry (MSn). Due to their selectivity, high sensitivity, multistage MS (MSn) capability and compact size QIT mass spectrometers are convenient tools for a wide range of applications.
The first mass spectrometer developed by J.J. Thompson was based on a magnetic sector analyzer. Magnetic sector mass spectrometers are the oldest type of mass spectrometers which are also alternatively called ‘spectrograph’ because they used photographic plates as ion detectors. Magnetic sector analyzers use a magnetic field to cause ion beams from the ion source to move in a circular motion. The ion beam travels through a magnetic field where the magnetic force is applied perpendicular to the direction of the ion travel path. According to physics law, this perpendicular acceleration force causes the ion beam to travel in a circular motion. So the magnetic sector analyzers bend the ion beam in an arc. The radius (r) of this arc may vary depending on several factors including momentum of the ion, charge of the ion (C), and strength of the magnetic field (B). A magnetic sector is solely able to separate ions according to their mass-to-charge ratio (m/z). But to achieve higher resolution and to perform tandem mass spectrometry, it is essential to use an electric sector in series with a magnetic sector. Mass spectrometers having combined magnetic and electrostatic sectors are alternatively referred to as double-focusing magnetic sector MS. In 1936 the first double-focusing magnetic sector MS was introduced by A. Dempster, K. Bainbridge, and J. Mattauch. Double-focusing magnetic sector MS was extensively used in the 1950s and 1960s since they could produce high-resolution data.
Electrostatic sector mass analyzer consists of two concentrically curved plates on which equal and opposite voltages are applied. As the ion beam travels through the analyzer, it gets bent due to the potential across the plates. The ions are deflected in the electric field where ions with the same kinetic energy are focused while ions with different kinetic energy are dispersed. The radius (r) of the ion flight orbit is determined by the kinetic energy of the ion (V) and the voltage (E) applied across the plates. Electrostatic sector mass analyzer separates ions based on their kinetic energy differences, so it works as a kinetic energy analyzer. On the other hand, the magnetic sector separates ions according to their momentum, which helps resolve ions accelerated to uniform kinetic energy. An electrostatic sector, when put in series with a magnetic sector, could give highly improved mass resolution. When an electrostatic sector and a magnetic sector are combined in a mass spectrometer, it is called a double-focusing mass spectrometer instrument.
Parameters of Mass Analyzer
Resolution (Resolving power)- In mass spectrometry, the resolution is defined as the ability of a mass analyzer to differentiate between ions that have different mass-to-charge ratios (m/z). Hence higher resolution indicates enhanced ability to distinguish between ions. Definition of resolution commonly expressed as the following equation: Resolution = M/ΔM, where M stands for m/z and ΔM stands for “full width at half maximum (FWHM)”.
Mass accuracy- The accuracy of a mass analyzer is defined as the ability to produce precise m/z information. Accuracy to a large extent is directly related to resolution and stability.
Mass range- This refers to the m/z range up to which a mass analyzer can scan. For example, a magnetic sector mass analyzer will scan up to m/z 10,000, a quadruple analyzer will scan up to m/z 3000, whilst time-of-flight (TOF) mass analyzers will have an almost unlimited m/z range.
Scan speed- Scan speed is also referred to as the scan rate. It is an indication of how fast a mass analyzer scans over a given mass range. Most of the currently available mass analyzers would perform a full scan within seconds, but it may significantly vary from one analyzer to analyzer. For example, time-of-flight (TOF) mass analyzers would take only milliseconds or less to complete a full scan.
Tandem mass spectrometry (MS/MS or MSn)- A tandem mass analyzer will have the ability to select (Precursor ions) then break them down into fragment ions (Product ions). By measuring the mass of fragment ions, the analyzer will determine the chemical structure of the original molecular ions (Precursor ions). In practice, a tandem mass analyzer is a combination of two analyzers.
Video: How Does Mass Spectrometer Work
Mass Spectrometers Easily Combine With Other Analytical Techniques. Here Are a Few Examples of Them.
Major Applications of Mass Spectrometry
Mass spectrometry is playing a crucial role in present-day scientific advancements such as studies of surface phenomena, international non-invasive monitoring of nuclear facilities, and astronomical research of solar systems.
Mass spectrometry is also playing a significant role in many commercial domains such as pharmaceutical industries, chemical and petrochemical industries, process monitoring in petroleum industries, textile industries, food processing industries, electronics industries, and carbon dating. It is also widely used in environmental analysis, pollution monitoring, forensic and toxicology analysis, drug of abuse diagnosis, clinical analysis, proteomics, metabolomics, imaging, glycomics, biomedical and biological applications.
Table: Typical Technical Specifications of Mass Spectrometer for GC
Parameter | Single Quadrupole MS | Tripple Quadrupole MS |
|---|
Scan methods
Standard Ionization mode
Optional Ionization mode
Ion source
Quadruple Ion guide (Q0)
Ion Source temperature
Quadrupole temperature
Filament and emission current
Electron energy
Mass filter
Collision cell
Not applicable
Mass range (m/z)
Scan speed
Mass accuracy
Related Reading