A vacuum system is a central element of a mass spectrometer. Several considerations require a mass spectrometer to have the capability to accomplish and sustain a robust vacuum within itself. An effective vacuum is a critical requirement for a mass spectrometer to achieve optimum performance and generate a high-quality mass spectrum. Here we will elaborate on how the vacuum environment influences different parameters of a mass spectrometer.
Contaminant free environment- The purpose of the vacuum is to free the analyzer environment from contaminants such as waters, chemicals, gases, and likes. Any molecule present within the mass analyzer will collide with sample ions. As a result of the collisions, ions might chemically react, neutralize, fragment, or scatter, which impacts the clarity of the mass spectrum. The sample ions' collision with gaseous atoms might also hinder their flight towards the detector. A high vacuum ensures that the mass analyzer gets rid of the air, water, chemicals, gases, and suspended particles to create an interference-free environment.
Arc free electric potential- A mass spectrometer performs sample analysis in a high electrical potential field. So, various components of the mass spectrometer will operate at high electrical potential creating a high voltage zone within the mass analyzer. A suitable vacuum wards off the possibility of arcing, helping sustain the required high electrical potential within the mass spectrometer.
Avoid loss of signal intensity- The vacuum level directly affects the output signal intensity of a mass spectrometer. For sustaining the signal strength, analyte ions mean free path must exceed the path length from the ion source to the detector. The mean free path of an ion refers to the average distance it travels between collisions. A high vacuum environment yields a longer mean free path than the vacuum chamber length resulting in smooth traveling of ions towards the detector. A sub-optimum vacuum will contain impurities such as air, moisture, and gas particles that might collide or react with analyte ions obstructing their flight and suppressing the signal strength. In conclusion, a lower than optimum vacuum causes a loss in mass spectrometer sensitivity resulting in a hazy mass spectrum.
What is a Vacuum System?
In simple words, a vacuum system represents a space whose pressure is below atmospheric pressure. The standard atmospheric pressure at sea level is 1.01325 bar which is equivalent to 1013.25 millibars, 14.696 psi, 101325 Pascal, 760 mm Hg, or 29.9212 inches Hg. Therefore any vacuum will have a lower pressure than this pressure. Reducing air and gas molecules from any space will cause the formation of a vacuum within that space. In other words, the differential pressure developed by removing air/gas molecules from a system constitutes a vacuum. Ideally, a vacuum is a space devoid of any matter. Depending on the applications vacuum system can be sealed type or opened type. A mass spectrometer requires a sealed type vacuum system. The units for measuring vacuum are Torr, Pascal (Pa), Millibar, etc.
Also read: A Guide to Optical Spectrometry
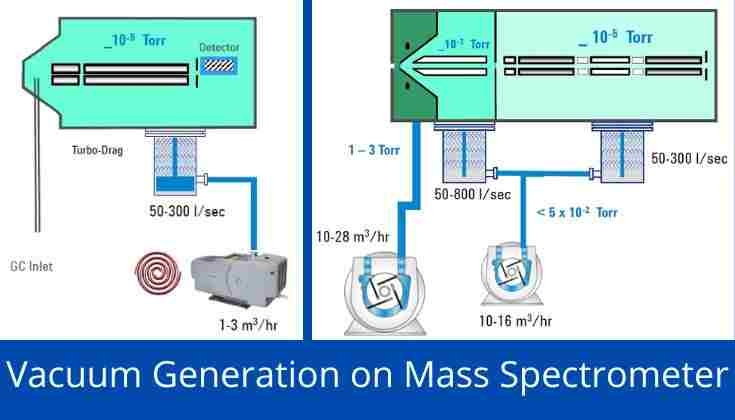
Vacuum System of Mass Spectrometer-
Rough Vacuum- The high vacuum of a mass spectrometer requires a two-stage pumping system. The first stage is a rough vacuum ranging from 1e3 mBar to 1e-3 mBar. Dry and oil-free mechanical pumps are preferable for this stage. A mass spectrometer uses this stage to remove carrier gas, atmospheric leakage, and gaseous contaminants. This stage may use one of the following pumps that are commonly known as roughing/foreline pumps:
High Vacuum- The second stage provides the actual high vacuum (HV), ultra-high vacuum (UHV), or extreme high vacuum (XHV) conditions. The vacuum range of the second stage is 1e-3 mBar to <1e-7 mBar. This stage may use one of the following pumps that can rapidly generate vacuum conditions by extracting gas molecules:
Vacuum Chamber of Mass Spectrometer-
The vacuum housing of a mass spectrometer is usually an inert stainless steel or aluminum chamber that operates under high vacuum pressure. It has an airtight design. The ion source, mass analyzer, and detector of a mass spectrometer operate within a vacuum inside the vacuum chamber. It means ionization, ion filtration, and detection of ions take place inside the vacuum chamber. If the vacuum chamber does not have proper airtight sealing, it will suck in air and external particles that interfere with analyte ions and eventually hit the detector. It will result in the mass spectrometer producing a crowdy mass spectrum instead of showing only the samples of interest. Therefore the more airtight the vacuum chamber of a mass spectrometer more precise is the resulting mass spectrum.
Related Reading
- What is Mass Spectrometry Technology? – A Beginner’s Guide
- General Technical Overview of Vacuum Ultraviolet Detector for Gas Chromatography – VUV Detector
- Technical Specifications of Gas Chromatograph with Flame Ionization Detector and Split/Splitless Inlet (GC with FID)
- An Informative Overview of Thermal Desorption (TD) Sample Introduction Technique for GC