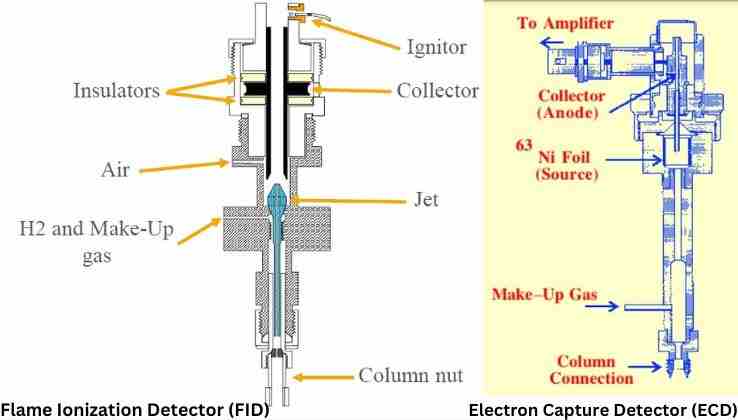
The detector of a chromatography system plays the vital function of detecting chromatographic information. A GC detector is a core constituent element of a gas chromatograph (GC) machine. It takes chromatographic information as input and generates output in suitable formats that humans can understand. The data given by GC detectors is recordable, storable in electronic memory, and printable. Humans can analyze and interpret stored or recorded GC detector data using computers and software to make crucial decisions.
The performance of gas chromatography detectors relies upon several technical parameters. The following are the different factors that influence the efficiency of a GC detector.
- Sensitivity
- Response Index
- Minimum Detection limit
- Linear dynamic range
- Precision
- Stability
- Reproducibility
- Resolution
- Noise level
- Pressure accuracy
- Flow accuracy
- Operating Temperature range
- Response time
- Data acquisition rate
A GC detector can be either destructive or non-destructive by design. A destructive GC-detector completely decomposes sample compounds, making them lose their physical and chemical properties and unusable for further analysis. With a non-destructive detector, sample compounds remain intact and usable with other detector types.
There are various types of GC detectors within the two main categories. Each GC detector works on unique principles and possesses different capabilities. The most common types of gas chromatography detectors are in the following list.
Destructive Gas Chromatography Detectors
- Flame Ionization Detector - FID
- Flame Photometric Detector – FPD
- Pulsed Flame Photometric Detector - PFPD
- Pulsed Discharge Helium Ionization Detector - PDHID
- Atomic-Emission Detector - AED
- Oxygenate Flame Ionization - O-FID
- Mass Spectrometry
Non-destructive Gas Chromatography Detectors
- Thermal Conductivity Detector - TCD
- Electron Capture Detector – ECD
- Photoionization Detector - PID
- Nitrogen-Phosphorus Detector - NPD (TSD)
- Olfactometric detector
- Sulfur Chemiluminescence Detector - SCD
- Nitrogen Chemiluminescence Detector - NCD
- Electrolytic Conductivity - ELCD
- Halogen Specific - XSD
There are some other gas chromatography detectors such as the following
First Generation of GC Detectors
- The Martin Gas Density Bridge
- Katharometer or hot wire detector (HWD)
- Flame Thermocouple Detector
- The β-Ray Ionization Detector
- The Emissivity Detector
- Macro Argon Detector
- Micro Argon Detector
- Thermal Argon Detector
- Discharge Ionization Detector
- The Radioactivity Detector
Less Known Gas Chromatography Detectors
- Thermionic Ionization Detector or Ionization Gauge Detector
- Discharge Detector
- Spark Discharge Detector
- Radio Frequency Discharge Detector
- Ultrasound Whistle Detector
- Dielectric Constant Detector
- Piezoelectric Adsorption Detector
- Absolute Mass Detector
- Surface Potential Detector
In the following section, we will elaborate on the working principles for each type of GC detector, spelling out their construction, features, specialties, strengths, drawbacks, and applications. So, we start here.
Flame Ionization Detector – FID
Flame Thermocouple Detector, developed by Dr. R.P.W. Scott (1924–2011), was the precursor to Flame Ionization Detector (FID). FID is the most commonly used detector in gas chromatography analysis. An FID detector comprises a hydrogen (H2) flame and an ion collector. The fuel of H2 flame is highly pure H2 gas or a mix of H2/He or H2/N2 and hydrocarbon free zero air. The flame of the GC fid detector operates in an electric field. The collector is a metal plate biased with a high DC negative voltage. FID GC detector offers extreme sensitivity, low detection limit, reliability, wide dynamic range, fast data acquisition, and lesser maintenance at affordable cost.
The analyte molecules that exit the GC column are fed into the flame inside the GC FID. The H2 flame breaks down sample gas molecules and ionizes their fragments. However, the GC-FID flame will not ionize the carrier gas. Widely used carrier gasses for the GC flame ionization detector are helium (He) and nitrogen (N2) which render almost zero ions in flame. The electrode of the fid flame ionization detector attracts the positively charged ions and produces a current proportional to the ionization rate that varies with analyte concentration. Subsequently, an electrometer transforms the collector current into electrical signals in millivolts or picoamperes.
GC FID responds to almost all organic compounds. Carbon-containing organic compounds easily ionize in hydrogen-air flame. So, the GC-FID is mainly used for hydrocarbon (HC) detection. However, FID doesn't ionize carbon sulfide, carbon monoxide, and carbon dioxide though they contain carbon. Also, gases like hydrogen, oxygen, sulfur dioxide, hydrogen sulfide, nitric oxide, nitrogen dioxide, ammonia, air, and water don't ionize in the flame, so FID doesn't respond to these compounds. The use of a Methanizer with a fid detector GC enables it to detect carbon monoxide (CO) and carbon dioxide (CO2) by catalytically converting them into methane (CH4).
Some industrial applications of the GC-FID method are as follows:
- Food, beverage, and cosmetic testing - headspace analysis of VOCs, profiling fatty acids in food
- Pharmaceutical testing - detecting residual contaminants
- Environmental monitoring - determining resin acids in water, measuring pollutants in the air
- Petrochemical analysis - measuring hydrocarbons
- Forensic testing - Identifying drugs of abuse, explosive
Limitations of Gas Chromatography Flame Ionization Detector (GC-FID)
- GC FID cannot analyze inorganic samples
- FID GC detector cannot detect analytes lacking carbon-hydrogen bonds
- FID detector gives poor response to compounds containing “heteroatoms” like oxygen
- FID destroys sample gas by oxidizing compound molecules as they pass thru the flame
Thermal Conductivity Detector – TCD
The thermal conductivity detector (TCD) is a widely used gas chromatography detector. TCD is among the first developed GC detectors. It is a non-destructive, universal detector, also known as Katharometer. TCD detector consists of two parallel cavities inside a heated metal block. The two cavities are equipped with four thickly coiled filaments (metal wire), each with two forming a Wheatstone bridge circuit. TCD works by sensing changes in the thermal conductivity of carrier gas as it absorbs heat from heated filaments while passing through the cavities.
Pure carrier gas follows through one cavity as a reference, while column effluent, a mix of vaporized solute and carrier gas, flows into the other cavity. The thermal conductivity of the carrier gas is constant. The thermal conductivity of most compounds is much lower than common carrier gases. Therefore, the presence of gaseous analyte molecules in carrier gas reduces the thermal conductivity of column effluent. The filament temperature is dependent on the thermal conductivity of the gas stream.
When column effluent interacts with the heated filament, it absorbs heat and decreases filament temperature, which changes filament resistance. As carrier gas and column effluent pass through the two cavities, a resistance difference develops between the two filament pairs due to the difference in heat conductivity between the two streams. The TCD detects the resistance difference and generates a signal which is a measure of the analyte concentration. So, GC-TCD is a differential technique that uses the thermal conductivity difference of carrier gas and sample.
A GC Thermal conductivity detector is relatively simple, reliable, sensitive enough for most jobs, has a linear response, and is robust. Traditionally helium or hydrogen is used as the carrier gas in GC TCD methods. Also, nitrogen is used as an alternative because helium is expensive. But with nitrogen as the carrier gas, there will be a high loss in TCD sensitivity. Hydrogen is dangerous.
Applications of Thermal Conductivity Detector in Gas Chromatography
- TCD is a general-purpose GC detector
- TCD detector in GC can detect both organic and inorganic samples
- It is responsive to almost any compound except the carrier gas
- It can analyze analytes to which FID has a weak response, hydrocarbons, permanent gases, inorganic gases
- It can detect air, water, hydrogen, nitrogen, carbon monoxide, carbon dioxide, argon, sulfur dioxide
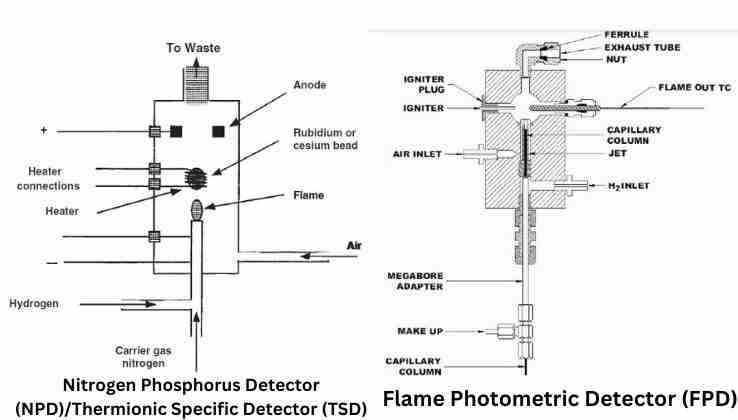
Flame Photometric Detector – FPD
Flame Photometric Detector (FPD) for GC works on a principle discovered by Draegerwerk for measuring phosphorus and sulfur compounds. The GC-FPD method has been in use since the 1960s. The main constituent elements of an FPD detector are a hydrogen-air flame, optical filters, and a photomultiplier tube (PMT). FPD is a GC detector specific and sensitive in detecting compounds containing sulfur (S) or phosphorous (P) and some metals. FPD is a destructive detector.
GC FPD excites analyte compounds to emit characteristic light via a photochemical process known as chemiluminescence emission. Then it photometrically detects the emitted light and converts it into an electrical signal. The intensity of the emitted light depends on the amount of sample. And the electrical signal intensity is a measure of analyte concentration.
Column effluent passes into the hydrogen-air flame of FPD. The H2-air flame decomposes sample compounds. Phosphorus (P) molecules break into HPO, while Sulfur (S) molecules break into S2. Under the flame's influence, HPO and S2 species get excited. When exited species return to the ground state, they emit light at specific wavelengths. HPO gives a maximum intensity of characteristics emission at 526 nm for phosphorus. S2 provides the highest intensity spectral emission at 394 nm for sulfur. Interference filters of narrow bandpass fitted between the flame and the PMT to isolate phosphorus and sulfur emission bands. Finally, the radiated light of 526 nm and 394 nm WL passed onto the PMT, which converts them into current and amplifies them to give detector signals. Interfering spectral emission bands are filtered out by optical filters. A poor thermal conductive shield protects the PMT and filters from the heated block.
GC FPD detector is slightly more responsive to phosphorus radicals than sulfur ones. The FPD gives a linear response to phosphorus compounds. However, its response to sulfur compounds is inherently non-linear. GC-FPD exponentially responds to sulfur molecules from 1 to 100 ppm. It can detect ppb-level phosphorus compounds and lesser than ppm-level sulfur compounds. FPD can measure up to sub-nanogram amounts of samples.
Applications of Gas Chromatography Flame Photometric Detector (FPD)
- GC-FPD method has wide use for the analysis of sulfur-containing compounds like hydrogen sulfide or sulfur dioxide, halogens-containing compounds, phosphorus-containing compounds
- FPD can detect heavy metals like chromium, arsenic, lead, iron, tin, and boron in organometallic compounds
- FPD can determine trace sulfur in hydrocarbons, gasoline
- FPD detector has extensive use for the analysis of pollutants in air and water, organic compounds containing oxygen, nitrogen, and chlorine, hetero-atoms, coal hydrogenation products
- FPD can determine sulfur in biological samples like amino acids, vitamins, some metalloproteins, alpha-lipoic acid, thiamine, and biotic
- FPD also detects phosphorus in biological samples like DNA, RNA, enamel, hydroxyapatite
Electron Capture Detector – ECD
Electron Capture Detector (ECD) was invented in 1957 by English chemist Dr. James Ephraim Lovelock (1919-2022), who was famed for designing scientific instruments. He was also a doctor and an author. An ECD detector features an ionization chamber with two biased electrodes set in parallel and a radioactive source. One electrode acts as collector anode and the other as cathode. The radioactive source is usually Nickel-63 (63Ni) of 10 mCi (millicuries) held in a metal foil or tritium. The radioactive nuclide emits beta particles (electrons). A potential difference across the electrodes enables a current flow between them. ECD is a Nondestructive GC detector.
Electrons emitted by the radionuclide collide with molecules of carrier gas (mobile phase) and ionize them. Ionization of the carrier gas produces a cloud of free electrons. These free electrons move fast toward a positively charged collector anode, causing a steady current flow between the electrodes. The constant current flow between electrodes acts as a background signal. Electronegative analyte molecules eluted from the column pass between the electrodes and absorb free electrons, decreasing the background current. The GC-ECD converts the current decrease into a positive signal, which represents a measure of the solute. The amount of captured electrons is proportional to the analyte concentration.
Electron Capture Detector sensitivity is 10 to 1000 times higher than FID and up to 100,000 times more than TCD. GC ECD can detect as low as 5.0 femtograms of compounds per second (fg/s). ECD is the first GC detector to measure parts-per-billion (ppb) and parts-per-trillion (ppt) levels of concentrations. It typically delivers a 10,000-fold linear range.
The extreme sensitivity of a GC ECD detector enables it to detect even trace impurities. GC-ECD method has a lot of applications in environmental control, food, pharmaceutical, forensic and other industries. ECD detector GC has wide use for the following processes.
- ECD has widespread use in the analysis of high electron affine compounds such as halogen-containing compounds, carbonyl compounds, nitro compounds, anesthetic gases, nitriles, organometallic substances, peroxides, benzodiazepines, chlorinated insecticides, fluorinated compounds, brominated compounds like bromoform
- ECD is used in environmental monitoring to determine herbicides, polychlorinated biphenyls (PCBs), halogenated pollutants, pesticides like DDT, organochlorine pesticides, chlorofluorocarbons (CFCs)
- ECD is used to analyze halogenated contaminants in food
- In drug testing, ECD is used to detect cannabinoids like CBD in fiber-type Cannabis and in hashish
- ECD can analyze nitrosourea compounds in brain tissue, methyltetrahydrophthalic anhydride in air, guaifenesin in human serum, iodopropynyl butyl carbamate in cosmetics, dibromoethane in gasoline, alpha-cypermethrin and deltamethrin in mosquito nets, trace explosive vapors
- ECD can determine organic chlorine-containing pesticides, phenoxy acid herbicides, and volatile chlorine-containing hydrocarbons in water
- ECD can analyze ethion and quinalphos in green pea
- ECD can detect chlorine-containing pesticides in aquatic tissue
- ECD can determine polychlorinated biphenyls (PCBs), organic chlorinated pesticides in solid waste, bottom sediment
- ECD can analyze sulfur dioxide, multi-class pesticide residues in wine
- ECD can detect femtogram levels of organically halogenated compounds, carbon tetrachloride
- ECD can detect parts per trillion (ppt) concentrations of SF6
There are some drawbacks of gas chromatography ECD detector such as the followings
- GC ECD detector cannot detect compounds like hydrocarbons, amines, and alcohols
- GC Electron Capture Detector exhibits a limited linear dynamic range, usually to the tune of two orders
Photoionization Detector - PID
A photoionization detector (PID) detects samples by ionizing sample molecules using ultraviolet (UV) light. A PID detector block consists of an ionization chamber, an ultraviolet light source, and two electrodes maintained at a potential difference. PID is a non-destructive GC detector. So, PID exhausts are usable for further analysis by other detector types in a multi-detector system.
Analyte molecules eluting from the GC column move into the analysis chamber of the photoionization detector. Inside the ionization chamber, sample molecules absorb ultraviolet light produced by the ultraviolet lamp. The absorbed UV light causes the molecules to eject electrons and become ionized molecules of positive charge. The ionization of gas molecules results in negatively charged electrons and positively charged molecular ions. The negative electrons and positive ions attracted by positive and negative electrodes generate a current flow between the electrodes. The intensity of the current is directly proportional to the quantity of ionized molecules. This current gives a measure of analyte concentration.
A Photo-ionization detector can deliver high sensitivity and produce a wide dynamic range (WDR). PIDs can detect a wide array of organic or inorganic vapors, spanning over 700 volatile organic compounds (VOC) species. PID detectors can measure VOCs and other gases from ppb to ppm concentrations. Photo ionization detectors are available as both GC detectors and portable handheld instruments.
The PID photoionization detector is widely employed to monitor VOCs and toxic gas exposure. Following are some of the applications of photoionization detectors.
- Detecting VOC leaks in industrial facilities, manufacturing processes, mining, military sites, waste management
- Measuring VOCs and other contaminants in air, water, and soil that include alcohols, aldehydes, acetone, organosulfur compounds, organic phosphonates, some organometallics, and others
- Measuring hazardous substances such as benzene, various solvents, spilled oil, lubricants, and degreasers in workplaces
- Analysis of e-cigarette vapor, ammonia, propylene, aromatic and aliphatic hydrocarbons, heterocyclics
- Analysis of organic volatiles present in human samples
- Monitoring of occupational health and safety
- Analysis of environmental toxins, some poison gases
Limitations of Photoionization Detector
- PID detector is not responsive to semi-volatile compounds
- Photo ionization detectors don't detect formaldehyde, methane, most chloro, fluoro, or bromo compounds
- PID cannot measure natural compounds having saturated bonds such as hydrogen, ingredients of air, acidic gases, acetylene, radioactive materials, ozone
Nitrogen Phosphorus Detector – NPD (TSD)
Nitrogen Phosphorus Detector (NPD) for gas chromatography was first introduced in the 1970s by B. Kolb and J. Bischoff. Then, Paul L. Patterson advanced the commercial use of the NPD detector. NPD is also known as a thermionic specific detector (TSD) due to its use of thermionic ionization of analytes and thermionic electron emission. Though the physical design of NPD appears almost identical to FID, the NPD's operating principle is entirely different from FID.
As the name suggests, NPD is a GC detector specific for nitrogen and phosphorus containing organic compounds. The response of the NPD detector is 100,000 times higher to nitrogen species and 1,000,000 times higher to phosphorous species than carbon.
GC NPD consists of a rubidium or cesium silicate bead placed within an electrically heated coil and mounted near the hydrogen flame. The heated coil heats the silicate bead that emits electrons by thermionic emissions. An electrode attracts the free electrons that create a flow of current serving as the base current. Here, nitrogen and phosphorus containing analyte molecules leaving the column get partially combusted in the hydrogen/air flame. These partially burnt molecules hit the bead and trigger chemical reactions that increase electron emissions from the heated bead. The increase in electron emission increases the current flow to the electrode. The increased current flow provides a measure of detector response that translates into chromatographic data. NPD uses a much lower flow of hydrogen in contrast to FID.
Applications of Nitrogen Phosphorus Detector
Common applications of GC NPD detector include the detection of compounds containing pesticides and herbicides, pharmaceutical analysis, drugs of abuse detection, forensic sample analysis, environmental analysis, and detection of toxic chemical vapors and other trace compounds.
Disadvantages of GC-NPD
- The bead has a fixed life; the bead's efficiency degrades over time, eventually ceasing to respond
- The bead needs periodic replacement
Purity of GC Carrier Gas
A GC carrier gas must contain a low percentage of water, oxygen, and hydrocarbon. Water and oxygen can react with the column stationary phase, cause column bleeding, and raise the baseline noise of the chromatogram. As a result, the column will have a short life, and system sensitivity will fall. Hydrocarbon also pushes up baseline noise, and GC detectors will lose sensitivity.
Related Reading
- Technical Specifications of Gas Chromatograph with Flame Ionization Detector and Split/Splitless Inlet (GC with FID)
- General Technical Overview of Vacuum Ultraviolet Detector for Gas Chromatography – VUV Detector
- An Informative Overview of Thermal Desorption (TD) Sample Introduction Technique for Gas Chromatography (GC)